Physicochemical and tribophysical properties of trioctylalkylammonium bis(salicylato)borate (N888n-BScB) ionic liquids: effect of alkyl chain length†
Abstract
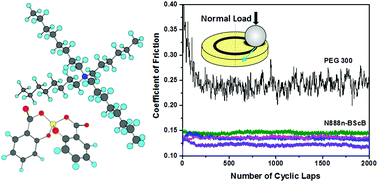
The alkyl chain length of trioctylalkylammonium bis(salicylato)borates (N888n-BScB; n = 6, 8, 10 and 12) was varied to prepare a series of room-temperature ionic liquids, and then their viscosity and rheological properties were investigated. Besides the omnipresent Coulombic interactions, other interactive forces such as van der Waals interactions, hydrogen bonding, inductive forces, dipole–dipole interactions, etc., collectively determine the physicochemical properties of N888n-BScB ionic liquids. The van der Waals interactions and structural geometry of the ammonium cation (N888n) primarily organized the packing orientation of N888n-BScB ionic liquids and controlled their viscosity and rheological properties as a function of the alkyl chain length. The symmetric cation (N8888) increased the viscosity owing to closer packing driven by van der Waals interactions. The N888n-BScB ionic liquids exhibited non-Newtonian shear thinning behaviour. Furthermore, the decrease in viscosity at higher shear rates indicated that interactive forces in the N888n-BScB ionic liquids were disrupted. These ionic liquids, as lubricants, exhibited significantly lower friction (40–50%) and wear (45–69%) in comparison to PEG 300 synthetic lubricating oil. The degrees of reduction in friction and wear were largely influenced by the chain length of the alkyl group. The N888n-BScB ionic liquids with longer alkyl chains were strongly adsorbed on sliding surfaces and provided better lubrication properties than those with shorter alkyl chains. As a result, the coefficients of friction and wear were decreased by increasing the chain length in N888n-BScB ionic liquids. The tribologically induced adsorption of the BScB anion on metal surfaces, electrostatic interactions between ions, the compact and rigid structure of the BScB anion and van der Waals interactions provided by longer alkyl chains in the N888n cation collectively formed a tribochemical thin film of low shear strength, which resulted in a reduction in friction and the avoidance of direct contact between the aluminium and steel tribopair.


 Please wait while we load your content...
Please wait while we load your content...