Effect of ferrous ion concentration on the kinetics of radiation-induced iron-oxide nanoparticle formation and growth
Abstract
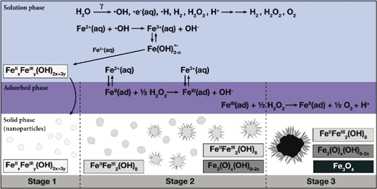
Magnetite nanoparticles were formed by γ-radiolysis of solutions containing different initial concentrations of FeSO4 without any other chemical additives. The particles formed in a given [Fe2+]0 had a narrow size distribution and the average size increased with [Fe2+]0. Five hour irradiation at 0.8 Gy s−1 produced an average size ranging from 23 ± 2 nm to 300 ± 40 nm in 0.1 mM or 10 mM [Fe2+]0 solutions, respectively. To ascertain the size-determining mechanism, the kinetics of γ-radiation-induced particle formation and growth were investigated by simultaneously analyzing the [H2(g)] in the headspace, the [FeII] and [FeIII] dispersed in solution, UV-Vis absorbances at 304 nm and 380 nm, and the pH of the solution. The particles formed were characterized by TEM imaging and various spectroscopic analyses. For a given [Fe2+]0 the time-dependent behaviours of different analyses collectively show three distinct kinetic stages of iron oxidation. The [Fe2+]0 affects the oxidation kinetics of different stages and hence, the oxidation yields and the size of particles formed after irradiation. The main processes which cause the observed kinetics and yields in the three stages are proposed.


 Please wait while we load your content...
Please wait while we load your content...