Synthon trends according to acid strength and geometry in salts of N-heterocyclic bases†
Abstract
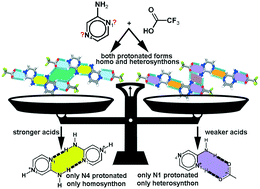
The hierarchy and robustness of homosynthons and heterosynthons formed by N-heterocyclic bases were assessed experimentally in salts of aminopyrazine (ampyz) and trans-1,2-bis(4-pyridyl)ethane (BPE) with common strong, moderate and weak acids, and theoretically at the M06-2X/6-31+G** level of theory. A trend for a base-pairing primary homosynthon to assemble in ampyz salts as the acid strength increases can be drawn. This homosynthon is present in chloride and bromide salts of ampyz, which is compatible with protonation at N4 and the formation of an accessory four-point heterosynthon engaging two (ampyz)+ cations and two halides. This robust synthon is also present in chloride, bromide and dihydrogen phosphate salts of BPE. Among all our BPE multicomponent crystal forms, it is not found only in the uncommon phosphoric acid cocrystal of the dihydrogen phosphate salt. When ampyz was crystallized with weaker acids such as trifluoroacetic, trichloroacetic and phosphoric acids, the primary homosynthon disappears gradually as the strength acid decreases. In the last two cases, this homosynthon is not found, but the trifluoroacetate salt of ampyz is found to have both the base-pairing primary homosynthon and the two-point heterosynthon with carboxylate. Both monoprotonated forms at N1 and N4 are found together in this structure. Another trend of N1 protonation in the presence of counterions from inorganic tetrahedral oxoacids such as isopropyl sulfuric and phosphoric acids is also outlined, regardless of their acid strength.


 Please wait while we load your content...
Please wait while we load your content...