Influence of pH and citrate on the formation of oxalate layers on calcite revealed by in situ nanoscale imaging†
Abstract
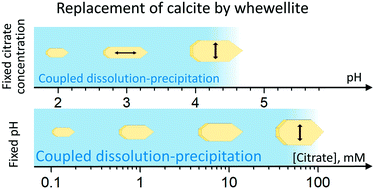
The influence of pH and citrate concentration on the replacement of calcite by calcium oxalate has been investigated by in situ nanoscale observations in flow-through experiments performed using atomic force microscopy (AFM). Significant changes in the morphology of the precipitated whewellite (CaC2O4·H2O) crystals were observed, as well as in the degree of interface coupling of the dissolution–precipitation reactions, depending on the pH of the solution. Citrate also influences whewellite morphology; different citrate species seem to preferentially adsorb on different whewellite faces according to our experimental observations. The results of this study may help in the design of effective treatments for the protection of calcareous stone, based on the natural process of patina formation that occurs in monuments, as well as to better understand the process of unwanted oxalate precipitate formation and its control by small organic molecules such as citrate.


 Please wait while we load your content...
Please wait while we load your content...