Preparation of hexagonal prism anatase with high thermal stability from a HTiOF3 precursor†
Abstract
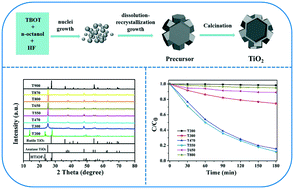
Anatase hexagonal prism TiO2 with high thermal stability was prepared through the decomposition of precursors (HTiOF3). HTiOF3 was firstly synthesized, using a solvothermal method, from n-octanol, tetrabutyl titanate and hydrofluoric acid. The obtained precipitates were then calcined at different temperatures (200–900 °C) for 2 h. The formation process of the hexagonal prism HTiOF3 precursors included three steps: nuclei growth, dissolution–recrystallization and further growth. The morphologies of the precursors depended strongly on the reaction temperature and the amount of solvent. The precursors were transformed into TiO2 after calcination and the effect of the calcination temperature on the morphology and phase composition of the hexagonal prism TiO2 was investigated. The results indicated that the anatase hexagonal prism TiO2 exhibited a rather high thermal stability, maintaining both the anatase structure and precursor shape up to 800 °C. The sample that was calcined at 550 °C exhibited a superior photocatalytic performance.

 Please wait while we load your content...
Please wait while we load your content...