Probing the role of the backbone carbonyl interaction with the CuA center in azurin by replacing the peptide bond with an ester linkage†
Abstract
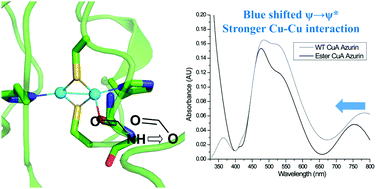
The role of a backbone carbonyl interaction with an engineered CuA center in azurin was investigated by developing a method of synthesis and incorporation of a depsipeptide where one of the amide bonds in azurin is replaced by an ester bond using expressed protein ligation. Studies by electronic absorption and electron paramagnetic resonance spectroscopic techniques indicate that, while the substitution does not significantly alter the geometry of the site, it weakens the axial interaction to the CuA center and strengthens the Cu–Cu bond, as evidenced by the blue shift of the near-IR absorption that has been assigned to the Cu–Cu ψ → ψ* transition. Interestingly, the changes in the electronic structure from the replacement did not result in a change in the reduction potential of the CuA center, suggesting that the diamond core structure of Cu2SCys2 is resistant to variations in axial interactions.

 Please wait while we load your content...
Please wait while we load your content...