A serendipitous cascade of rhodium vinylcarbenoids with aminochalcones for the synthesis of functionalized quinolines†
Abstract
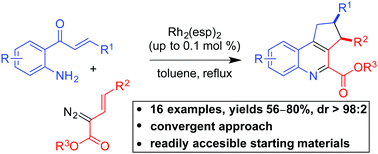
A serendipitous five-step cascade of rhodium vinylcarbenoids with aminochalcones enables a unique synthetic approach to highly functionalized tri- and tetra-cyclic quinolines. The cascade reaction begins with the insertion of aminochalcone nitrogen into rhodium vinylcarbenoids followed by intramolecular aldol cyclization to provide a substituted indoline intermediate that undergoes an oxy-Cope rearrangement to provide a 9-membered azacycle, which then rearranges to the functionalized quinoline through an intramolecular aldol/dehydration sequence. With a catalyst loading as low as 0.1 mol%, the cascade reaction has proven to be general with a range of aminochalcones and vinylcarbenoids.


 Please wait while we load your content...
Please wait while we load your content...