The first synthesis of meso-dicycloalkylporphycenes: ring strain effects on structural and optical properties of isomeric porphyrins†
Abstract
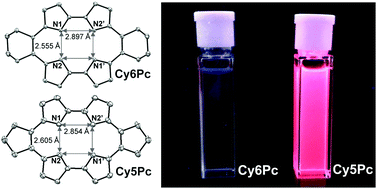
Two novel meso-dicycloalkylporphycenes, namely meso-dicyclopentyl- (Cy5Pc) and meso-dicyclohexylporphycenes (Cy6Pc) are synthesized for the first time in porphycene chemistry. We use intermolecular oxidative coupling of 5,6-dicycloalkydipyrroethenes mediated by hypervalent iodine(III) reagents. The solution fluorescence maxima of Cy5Pc are more than 400 times as intense as that of Cy6Pc. The results suggested that photoluminescence switching is achieved through the ring strain perturbation of the meso-substituted cycloalkyl groups on the porphycene cavity.


 Please wait while we load your content...
Please wait while we load your content...