Computationally-guided optimization of small-molecule inhibitors of the Aurora A kinase–TPX2 protein–protein interaction†
Abstract
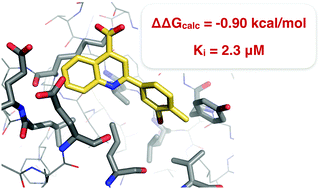
Free energy perturbation theory, in combination with enhanced sampling of protein–ligand binding modes, is evaluated in the context of fragment-based drug design, and used to design two new small-molecule inhibitors of the Aurora A kinase–TPX2 protein–protein interaction.


 Please wait while we load your content...
Please wait while we load your content...