Ring closing metathesis of unprotected peptides†
Abstract
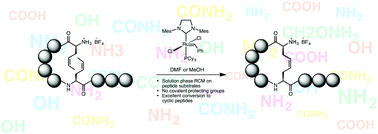
An efficient and expedient route to the synthesis of dicarba peptides from protecting group-free sequences is reported using Ru-alkylidene catalysed olefin metathesis. A range of cyclic peptides was prepared from linear peptides containing two Z-crotyl glycine residues. Free amine groups were masked as salts with Brønsted acids preventing in situ catalyst decomposition. Excellent RCM conversion was obtained in both DMF and methanol.


 Please wait while we load your content...
Please wait while we load your content...