Pd(0)-Catalyzed intramolecular arylative dearomatization of β-naphthols†
Abstract
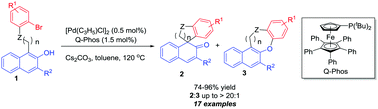
An efficient Pd(0)-catalyzed intramolecular arylative dearomatization of β-naphthols is described. Using Q-Phos as a ligand, the arylative dearomatization reaction proceeded smoothly affording excellent yields and chemoselectivity even when the catalyst loading was reduced to 0.1 mol%. This method offers an efficient access to a series of structurally diverse spirocarbocycles. Preliminary investigation indicates that an enantioselective reaction is feasible in the presence of a chiral phosphoramidite ligand.


 Please wait while we load your content...
Please wait while we load your content...