SNAr catalysis enhanced by an aromatic donor–acceptor interaction; facile access to chlorinated polyfluoroarenes†
Abstract
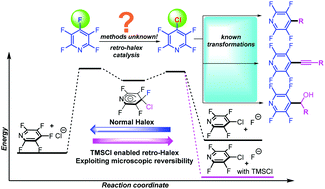
Selective catalytic SNAr reaction of polyfluoroaryl C–F bonds with chloride is shown. Stoichiometric TMSCl makes the reaction exergonic and allows catalysis, which involves ground state elevation of chloride, aromatic donor–acceptor interactions, and stabilization of the Meisenheimer complex. Traditional cross-coupling of the products is now possible and demonstrates the utility.


 Please wait while we load your content...
Please wait while we load your content...