Metal-free radical oxidative alkoxycarbonylation and imidation of alkanes†
Abstract
A metal-free radical oxidative carbonylation of alkanes is demonstrated, yielding esters and imides by means of di-tert-butylperoxide as an oxidant. Various alkanes, alcohols and amides were compatible in this system generating the desired carbonyl products in up to 86% yields. We proposed a plausible radical cross-coupling process based on the preliminary mechanistic studies.
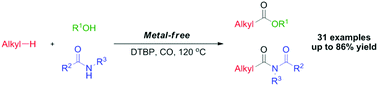


 Please wait while we load your content...
Please wait while we load your content...