Ruthenium(ii)-catalyzed intermolecular synthesis of 2-arylindolines through C–H activation/oxidative cyclization cascade†
Abstract
Herein, we report a ruthenium-catalyzed 1,2-carboamination through C–H activation for the synthesis of 2-arylindolines from readily available, inexpensive, protected anilines and vinyl arenes. In earlier reports, indoline synthesis through C–H activation was demonstrated using sterically or electronically biased olefins suppressing β-hydride elimination. However, in the present protocol a ruthenium(II)-catalyzed indoline synthesis via interrupted Heck-type manifold with unbiased styrenes is demonstrated. Mechanistically, the reaction proceeds through pyrimidine directed electrophilic ortho metalation, alkene insertion, amine coordination, and reductive elimination to construct the indoline nucleus.
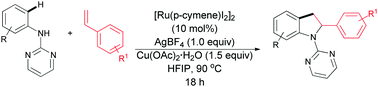


 Please wait while we load your content...
Please wait while we load your content...