Rapid assembly of the doubly-branched pentasaccharide domain of the immunoadjuvant jujuboside A via convergent B(C6F5)3-catalyzed glycosylation of sterically-hindered precursors†
Abstract
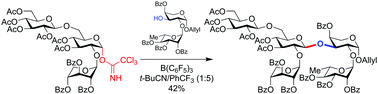
A convergent synthesis of the complex, doubly-branched pentasaccharide domain of the natural-product immunoadjuvant jujuboside A is described. The key step is a sterically-hindered glycosylation reaction between a branched trisaccharide trichloroacetimidate glycosyl donor and a disaccharide glycosyl acceptor. Conventional Lewis acids (TMSOTf, BF3·Et2O) were ineffective in this glycosylation, but B(C6F5)3 catalyzed the reaction successfully. Inherent complete diastereoselectivity for the undesired α-anomer was overcome by rational optimization with a nitrile solvent system (1 : 5 t-BuCN/CF3Ph) to provide flexible, effective access to the β-linked pentasaccharide.


 Please wait while we load your content...
Please wait while we load your content...