Synthesis and characterization of a dipyriamethyrin–uranyl complex†
Abstract
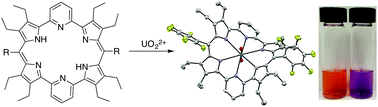
The reaction between non-aqueous uranyl silylamide (UO2[N(SiMe3)2]2·2THF) under anaerobic conditions or uranyl acetate (UO2(OAc)2·2H2O) under standard laboratory conditions and dipyriamethryin affords a bench-stable uranyl complex. Competition studies as well as DFT calculations provide support for the observed selectivity for the uranyl cation over trivalent lanthanide and multiple transition metal precursors.


 Please wait while we load your content...
Please wait while we load your content...