A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture†
Abstract
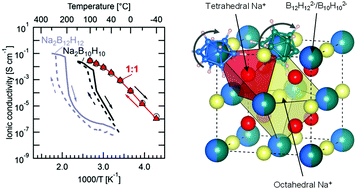
Na2(B12H12)0.5(B10H10)0.5, a new solid-state sodium electrolyte is shown to offer high Na+ conductivity of 0.9 mS cm−1 at 20 °C, excellent thermal stability up to 300 °C, and a large electrochemical stability window of 3 V including stability towards sodium metal anodes, all essential prerequisites for a stable room-temperature 3 V all-solid-state sodium-ion battery.


 Please wait while we load your content...
Please wait while we load your content...