Pyreneacyl sulfides as a visible light-induced versatile ligation platform†
Abstract
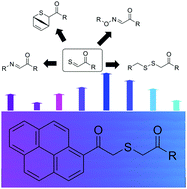
We report a visible light responsive moiety capable of generating highly reactive thioaldehydes. Upon irradiation with visible light (420 nm) the pyreneacyl sulfide species undergoes a Norrish II elimination yielding thioaldehydes capable of being trapped by nucleophiles (amines, aminoxys, and thiols), as well as Diels–Alder processes, representing a new versatile ligation platform.


 Please wait while we load your content...
Please wait while we load your content...