A bidirectional synthesis of spiroacetals via Rh(ii)-catalysed C–H insertion†
Abstract
Acyclic methylene acetals bearing two diazoester subunits have been converted to [5,5]-spiroacetals VIA bidirectional C–H insertion under Rh(II) catalysis. Using a chiral Rh(II) catalyst, the major diastereomer can be produced in high enantiomeric excess (89%).
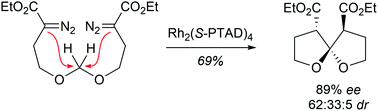


 Please wait while we load your content...
Please wait while we load your content...