Copper-catalyzed regioselective 1,2-thioamidation of alkenes†
Abstract
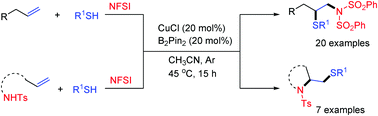
An efficient method for regioselective 1,2-thioamidation of terminal alkenes, catalyzed by copper in the presence of NFSI and thiols, has been disclosed. Under the reaction conditions, the amido radical was formed initially, followed by addition to the terminal carbon of alkenes and trapping of the resulting cuprate intermediate with thiols. Therefore, the regioselectivity was completely reversed compared to previously reported reactions which were initiated by sulfur-centred radicals or cations. When alkenes containing an amido N–H moiety were applied as substrates, intramolecular amido radical cyclization took place to afford sulfane substituted N-heterocycles.


 Please wait while we load your content...
Please wait while we load your content...