Facile one-pot synthesis of 2,3-dihydro-1H-indolizinium derivatives by rhodium(iii)-catalyzed intramolecular oxidative annulation via C–H activation: application to ficuseptine synthesis†
Abstract
Various substituted indolizidinium, quinolizinium and pyrido[1,2-a]azepinium salts synthesized from benzaldehydes (or α,β-unsaturated aldehydes) and alkyne–amines catalyzed by rhodium complexes via C–H activation are demonstrated. The reaction was carried out under mild reaction conditions using Cu(BF4)2·6H2O as oxidant and anion source and inexpensive oxygen as a co-oxidant. A reaction mechanism involving imine formation followed by an ortho C–H activation, alkyne insertion and reductive elimination via a 7-membered rhodacycle is proposed. The present method has been successfully applied to the synthesis of a natural product, ficuseptine.
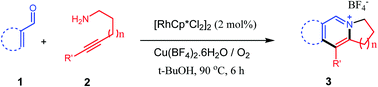


 Please wait while we load your content...
Please wait while we load your content...