6-exo-trig Michael addition-lactonizations for catalytic enantioselective chromenone synthesis†
Abstract
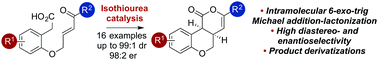
The catalytic enantioselective 6-exo-trig Michael addition-lactonization of enone-acid substrates to form cis-chromenones with high diastereo- and enantiocontrol was developed using the commercially available isothiourea tetramisole. An acidic workup proved necessary to minimize product epimerization and maximize product er, providing cis-chromenones in excellent yield, and with excellent diastereo- and enantioselectivity.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners


 Please wait while we load your content...
Please wait while we load your content...