meso-N-Methylation of a porphyrinoid complex: activating the H-atom transfer capability of an inert ReV(O) corrolazine†
Abstract
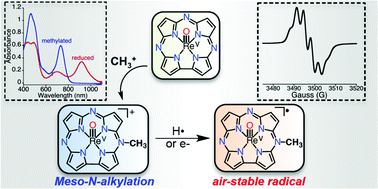
The selective alkylation of a single meso-N atom of a corrolazine macrocycle is reported. Alkylation has a dramatic impact on the physicochemical properties of ReV(O)(TBP8Cz). New electron-transfer and hydrogen-atom-transfer reactivity is also seen for this complex, including one-electron reduction, which gives an air-stable 19π-electron aromatic radical complex.


 Please wait while we load your content...
Please wait while we load your content...