In situ generation and reactions of p-(trifluoromethyl)benzyl electrophiles: an efficient access to p-(trifluoromethyl)benzyl compounds†
Abstract
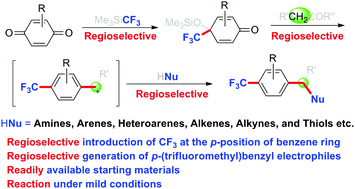
A new three-component reaction, namely condensation–anti-Michael addition–aromatization, enabling the construction of benzylic compounds is disclosed. This reaction can not only act as an alternative approach to regioselective Csp2–H trifluoromethylation of arenes through an “aromatic to be” strategy, but also provides a simple, convenient, step-economic, and practical strategy for the in situ generation of electrophilic p-(trifluoromethyl)benzyl species under extremely mild conditions.


 Please wait while we load your content...
Please wait while we load your content...