Kinetic analysis of copper transfer from a chaperone to its target protein mediated by complex formation†
Abstract
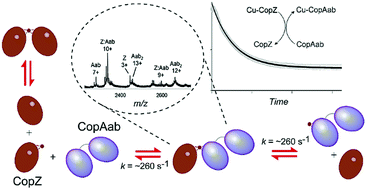
Chaperone proteins that traffic copper around the cell minimise its toxicity by maintaining it in a tightly bound form. The transfer of copper from chaperones to target proteins is promoted by complex formation, but the kinetic characteristics of transfer have yet to be demonstrated for any chaperone-target protein pair. Here we report studies of copper transfer between the Atx1-type chaperone CopZ from Bacillus subtilis and the soluble domains of its cognate P-type ATPase transporter, CopAab. Transfer of copper from CopZ to CopAab was found to occur rapidly, with a rate constant at 25 °C of ∼267 s−1, many orders of magnitude higher than that for Cu(I) dissociation from CopZ in the absence of CopAab. The data demonstrate that complex formation between CopZ and CopAab, evidence for which is provided by NMR and electrospray ionisation mass spectrometry, dramatically enhances the rate of Cu(I) dissociation from CopZ.


 Please wait while we load your content...
Please wait while we load your content...