Palladium-catalysed mono-α-alkenylation of ketones with alkenyl tosylates†
Abstract
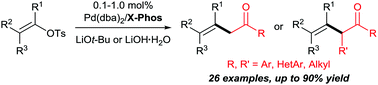
The first example of palladium-catalysed selective mono-α-alkenylation of ketones with alkenyl tosylates is described. In the presence of a Pd/XPhos catalyst system (0.1–1.0 mol%), the reaction provides mono-α-alkenylated ketones in good yields and exhibits excellent substrate tolerance. Highly congested, tri- and tetra-substituted alkenyl tosylates react smoothly and even problematic heteroaryl and aliphatic ketones are applicable substrates. Notably, small β,γ-unsaturated ketones are successfully prepared using acetone as a simple three-carbon feedstock.


 Please wait while we load your content...
Please wait while we load your content...