Characterization of phenolic acids binding to thrombin using frontal affinity chromatography and molecular docking
Abstract
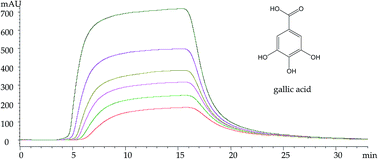
As a serine protease, thrombin (THR) plays an important role in the coagulation cascade. In this study, a frontal affinity chromatography (FAC) method was developed for the characterization of interactions between immobilized thrombin and phenolic acids, which have potential thrombin inhibitory activity. First, a simple method to immobilize thrombin was developed, where an immobilized artificial membrane (IAM) chromatographic column was used as the carrier material for entrapping THR. Then the frontal analysis of five compounds was performed both on the THR-IAM column and argatroban-THR-IAM column. Finally, the dissociation constants were determined and the values are 2.46, 12.42, 44.93, 76.91 and 100.69 μmol L−1 for gallic acid, protocatechuic acid, ferulic acid, chlorogenic acid and sinapic acid, respectively. In addition, molecular docking was further applied to study the interaction between the compounds and thrombin, and the rank order of docking energy values is similar to that of the dissociation constants of the compounds determined by FAC. The results of the present study demonstrate that the interaction between the compounds and thrombin can be well characterized by FAC experiments along with molecular docking.


 Please wait while we load your content...
Please wait while we load your content...