Profiling, characterization, and analysis of natural and synthetic acylsugars (sugar esters)
Abstract
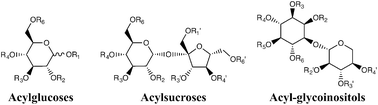
Acylsugars are an underappreciated group of specialized metabolites and synthetic products comprised of a sugar core esterified to one or more carboxylic acids at specific positions on the ring. Synthetic acylsugars have a wide array of commercial applications as food additives and preservatives, emulsifiers, cosmetic ingredients, plasticizers, and non-ionic surfactants, as well as in the formulation and delivery of drugs. In nature, acylsugars are specialized metabolites that are best known for accumulating in glandular trichomes of the plant family Solanaceae (tomato and its wild relatives and the genera Nicotiana and Datura), and evidence suggests they play important roles in plant chemical defenses against insects and other herbivores. Analysis and characterization of acylsugars involve: (a) purity assessment, (b) structural characterization of the sugar cores and the carboxylic acid esterified to the cores, (c) analysis of the number of acyl groups and their positions of esterification, and (d) quantitative determination. This review briefly describes and compares historical and current analytical strategies for acylsugar profiling including GC and GC/MS, nuclear magnetic resonance (NMR) spectroscopy, enzymatic transformations, and other liquid chromatography and mass spectrometry based approaches for analysis, characterization, localization, and quantification of acylsugars.


 Please wait while we load your content...
Please wait while we load your content...