Transition dipole moment orientation in films of solution processed fluorescent oligomers: investigating the influence of molecular anisotropy†
Abstract
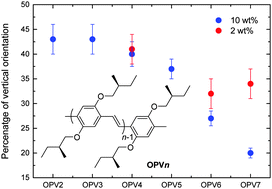
The low light-outcoupling efficiency of organic light emitting diodes (OLEDs) is limiting their performance. Orientation of the transition dipole moment of the emitting molecules in the plane of the diodes can improve the luminance of OLEDs. While the orientation of evaporated small-molecule materials has been studied in the past few years, not much is known about solution processed small molecules and short oligomers, and it is not clear yet which parameters influence their orientation in the film. In this work we study a series of short conjugated p-phenylene vinylene oligomers (OPVn), consisting of an increasing number of repeating phenyl rings (n from 2 to 7), which are introduced into a small-molecule host matrix. By measuring the angular distribution of p-polarised fluorescence intensity from thin solution processed films, we determine the average orientation of the transition dipole moment of the emitters in the host matrix. We find that for longer oligomers (n = 6, 7), the transition dipole moments align more horizontally, with ratios of horizontally to vertically oriented dipoles up to 80 : 20. The preferential horizontal alignment is related to the aggregation of the emitter molecules.

 Please wait while we load your content...
Please wait while we load your content...