Structure–function relationship exploration for enhanced thermal stability and electro-optic activity in monolithic organic NLO chromophores†
Abstract
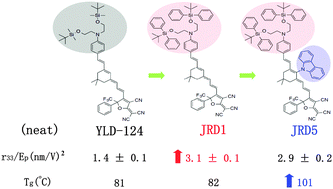
We have developed a series of novel monolithic materials based on molecules previously explored as dopants in guest–host systems to study intrinsic structure–function relationships in organic electro-optic (EO) materials. In a library of EO molecules with varied bridge segments, molecular modification of the donor with bis(tert-butyldiphenylsilyl) groups led to improvement in formation of amorphous films and led to enhanced poling efficiency. Further modification to include a carbazole site-isolation group on the bridge effectively reduced intermolecular dipole–dipole interactions, led to a material with poling efficiency of approximately 3 (nm V−1)2, and an increased glass transition temperature to 20–40 °C higher than similar reported monolithic materials. This level of thermal stability is comparable to common guest/host systems, which incorporated poly(methyl methacrylate) (PMMA) as the host. Our research showed that π-bridge length and type impacted first molecular hyperpolarizability β of a chromophore, which is accordingly reflected in the EO response. These findings further promote the utility of monolithic materials for their increased EO behavior and improved thermal stability, making this material system a competitor of guest–host systems in commercial applications.


 Please wait while we load your content...
Please wait while we load your content...