The end-capped group effect on dithienosilole trimer based small molecules for efficient organic photovoltaics†
Abstract
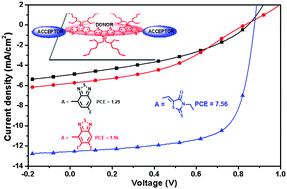
Three new small molecules, FBT–tDTS, DFBT–tDTS and RHO–tDTS, were designed and synthesized by using a rigid dithienosilole trimer (tDTS) as the novel donor unit and 5-fluorobenzothiadiazole (FBT), 5,6-difluorobenzothiadiazole (DFBT) and 3-ethylrhodanine (RHO) as acceptor units, respectively. According to the density functional theory calculations, RHO–tDTS has a more rigid structure due to the linkage between the donor and acceptor units. As a result, RHO–tDTS exhibits a red-shifted absorption spectrum and a higher hole mobility in comparison with FBT–tDTS and DFBT–tDTS. Small molecule solar cells based on RHO–tDTS show a power conversion efficiency of 7.56%, significantly performing better than the other two analogues. The mechanism of the good photovoltaic performance of RHO–tDTS was discussed. We also demonstrated that the end-capped groups on small molecules play an important role in tuning the performance of the organic photovoltaic devices.

 Please wait while we load your content...
Please wait while we load your content...