On the design of atropisomer-separable photochromic diarylethene-based metal–organic framework linkers†
Abstract
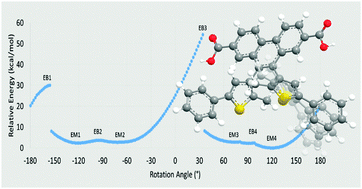
The relative stability and accessibility of atropisomers plays a prominent role in the efficacy of diarylethene-based photochromic materials. Herein, DFT methods, using the ωB97XD functional and a 6-31G(d) basis, are employed to determine the local energetic minima and maxima which describe the rotation of a thiophene group in derivatives of 9,10-bis(2-methyl-5-pheylthiophen-3-yl)phenanthrene-2,7-dicarboxylic acid, a photochromic linker molecule which exhibits atropisomer-formation-related fatigue when incorporated into a metal–organic framework. Results of these potential energy surface mapping calculations as well as their applications to atropisomer separability are discussed.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...