Perovskite oxides for application in thermochemical air separation and oxygen storage†
Abstract
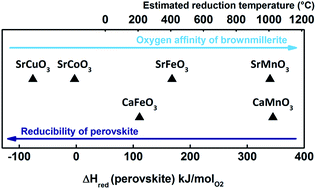
Perovskites AMO3−δ are ideal for thermochemical air separation due to their oxygen nonstoichiometry δ, which can be varied by changing the temperature and oxygen partial pressure. We show in this work how materials can be selected for chemical looping air separation from thermodynamic considerations and present thermogravimetric experiments carried out on (Ca,Sr) ferrites and manganites, and doped variants, all synthesized via a citric acid auto-combustion method. SrFe0.95Cu0.05O3−δ and Ca0.8Sr0.2MnO3−δ show the best gravimetric oxygen storage capacity of all tested materials at T < 1200 °C. The redox reactions are completed in <1 min in air and are highly reversible. A significant re-oxidation reaction of reduced samples was observed at temperatures as low as 250 °C at an oxygen partial pressure of 0.16 bar. We studied phase formation via XRD and lattice expansion during reduction via in situ XRD experiments. The objective is to validate the potential and boundary conditions of such materials to pave the way for competitive air separation based on thermochemical cycling.

 Please wait while we load your content...
Please wait while we load your content...