Transition metal dissolution and deposition in Li-ion batteries investigated by operando X-ray absorption spectroscopy†
Abstract
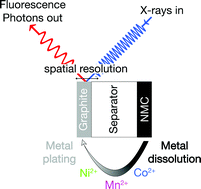
In Li-ion batteries the dissolution of transition metals from the cathode and their subsequent deposition on the anode are known to contribute to capacity fading. In this study, we investigate these processes using an NMC cathode and a graphite anode under operating conditions using X-ray absorption spectroscopy. The experiments are carried out in an operando cell, which allows both the time/voltage and spatially resolved determination of metal concentration and oxidation state of transition metal deposits on the graphite electrode. NMC shows a strong increase of the metal dissolution rate, if the upper cut off potential exceeds 4.6 V. Under operating conditions, the oxidation state of manganese, cobalt and nickel are found to be always +2 both on lithiated and delithiated graphite. In contrast, manganese is found to be present in the metallic state on lithiated graphite in the ex situ analysis, thus highlighting the importance of the operando characterization.

 Please wait while we load your content...
Please wait while we load your content...