Variation in surface energy and reduction drive of a metal oxide lithium-ion anode with stoichiometry: a DFT study of lithium titanate spinel surfaces†
Abstract
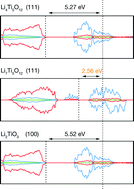
Li4Ti5O12 is a “zero-strain” lithium-ion anode material that shows excellent stability over repeated lithium insertion–extraction cycles. Although lithium (de)intercalation in the bulk material has been well characterised, our understanding of surface atomic-scale-structure and the relationship with electrochemical behaviour is incomplete. To address this, we have modelled the Li4Ti5O12 (111), Li7Ti5O12 (111) and α-Li2TiO3 (100), (110), and (111) surfaces using Hubbard-corrected density-functional theory (GGA+U), screening more than 600 stoichiometric Li4Ti5O12 and Li7Ti5O12 (111) surfaces. For Li4Ti5O12 and Li7Ti5O12 we find Li-terminated surfaces are more stable than mixed Li/Ti-terminated surfaces, which typically reconstruct. For α-Li2TiO3, the (100) surface energy is significantly lower than for the (110) and (111) surfaces, and is competitive with the pristine Li7Ti5O12 (111) surface. Using these stoichiometric surfaces as reference, we also model variation in Li surface coverage as a function of lithium chemical potential. For Li4Ti5O12, the stoichiometric surface is most stable across the full chemical potential range of thermodynamic stability, whereas for Li7Ti5O12, Li deficient surfaces are stabilised at low Li chemical potentials. The highest occupied electronic state for Li7Ti5O12 (111) is 2.56 eV below the vacuum energy. This is 0.3 eV smaller than the work function for metallic lithium, indicating an extreme thermodynamic drive for reduction. In contrast, the highest occupied state for the α-Li2TiO3 (100) surface is 4.71 eV below the vacuum level, indicating a substantially lower reduction drive. This result demonstrates how stoichiometry can strongly affect the thermodynamic drive for reduction at metal-oxide-electrode surfaces. In this context, we conclude by discussing the design of highly-reducible metal-oxide electrode coatings, with the potential for controlled solid-electrolyte-interphase formation via equilibrium chemistry, by electrode wetting in the absence of any applied bias.


 Please wait while we load your content...
Please wait while we load your content...