Anionic porous polymers with tunable structures and catalytic properties†
Abstract
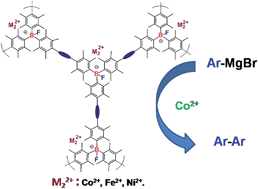
A series of boron-containing conjugated microporous polymers with hierarchical porous structures have been readily prepared via typical transition metal-catalyzed coupling reactions. The distribution of micro- and mesopores in the networks as well as the specific surface areas are tunable via tailoring the structures of the building blocks. The distinct capability of the resulting Lewis acid-based neutral porous polymers to selectively capture fluoride ions provides a high-efficiency conversion into stable anionic porous polymers. For the first time, fluoride anion binding to boron atoms in a solid sample was essentially characterized by solid-state 11B MAS NMR spectroscopy, clearly revealing such an efficient conversion from a neutral network to a negatively charged one only through Lewis acid–base adduct formation. Upon a simple ion-exchange process, various heavy metal cations were facile to be loaded into the networks of the anionic porous polymers. Furthermore, the cobalt(II)-loaded porous polymers were shown to promote the stoichiometric homocoupling reactions of the different aryl Grignard regents, and exert distinct size selectivities for the homocoupling products, highly dependent on their porous structures. Such a successful loading strategy might be used for design and synthesis of new types of zeolite-like porous polymers with desirable catalytic properties for a certain organic transformation, as well as other functional materials.


 Please wait while we load your content...
Please wait while we load your content...