Ultrasmooth metal halide perovskite thin films via sol–gel processing†
Abstract
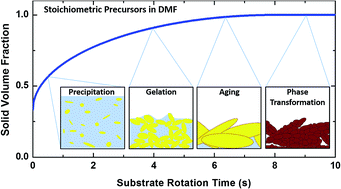
We demonstrate that lead halide perovskite thin film formation displays the characteristics of a sol–gel process. By performing a solvent exchange at different times, the stages of the sol–gel process are elucidated and their sensitivity to processing conditions are examined. For CH3NH3PbI3, the reaction and aging kinetics are found to be extremely rapid, complete within 10 s, and a competing formation of a highly crystalline PbI2:N,N-dimethylformamide (DMF) complex introduces additional complications relative to a well-behaved sol–gel process. Perovskite formation from strongly polar solvents can be described, in most cases, by the sol–gel processing of PbI2 regarding other solution components as additives. Understanding the details of additive and environmental influences on the sol–gel properties allow us to exploit fundamental concepts of sol–gel engineering to direct solvent and surfactant choices to control particle size and demonstrate multiple widely employed lead halide perovskite films (CH3NH3PbI3, CH3NH3PbBr3, and CsPbBr3) with unprecedented surface roughness of less than 2 nm.


 Please wait while we load your content...
Please wait while we load your content...