Waste PET (bottles) as a resource or substrate for MOF synthesis†
Abstract
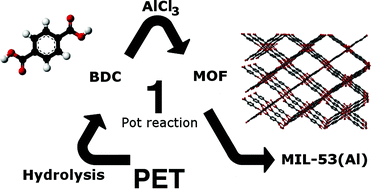
PET contains up to 85 wt% of terephthalic acid (BDC), but has never directly been used as a source of organic linker for MOF synthesis. By combining metal salts and PET under hydrothermal conditions in a microwave oven, PET hydrolysis and MOF synthesis occur simultaneously. With this one-pot reaction, MIL-53(Al) and MIL-47(V) have been successfully synthesized. Optimization of the reaction and activation conditions for MIL-53(Al) results in a phase-pure MOF with a BET surface of 1481 m2 g−1. When the hydrolysis is carried out as a separate first step, less stable MOFs like MIL-88B(Fe) can be synthesized by adding the metal salt and methanol to the hydrolyzed mixture in the second step. By partially depolymerizing the surface of PET bottles it is possible to grow MOF coatings of MIL-53(Al) and UiO-66(Zr) on the polymer surface, using the bottle itself as the synthesis reactor.

 Please wait while we load your content...
Please wait while we load your content...