Structure and dehydration mechanism of the proton conducting oxide Ba2In2O5(H2O)x
Abstract
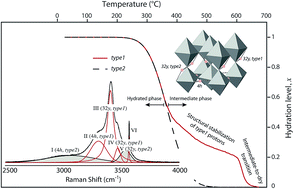
The structure and dehydration mechanism of the proton conducting oxide Ba2In2O5(H2O)x are investigated by means of variable temperature (20–600 °C) Raman spectroscopy together with thermal gravimetric analysis and inelastic neutron scattering. At room temperature, Ba2In2O5(H2O)x is found to be fully hydrated (x = 1) and to have a perovskite-like structure, which dehydrates gradually with increasing temperature and at around 600 °C the material is essentially dehydrated (x ≈ 0.2). The dehydrated material exhibits a brownmillerite structure, which is featured by alternating layers of InO6 octahedra and InO4 tetrahedra. The transition from a perovskite-like to a brownmillerite-like structure upon increasing temperature occurs through the formation of an intermediate phase at ca. 370 °C, corresponding to a hydration degree of approximately 50%. The structure of the intermediate phase is similar to the structure of the dehydrated material, but with the difference that it exhibits a non-centrosymmetric distortion of the InO6 octahedra that is not present in the dehydrated material. The dehydration process upon heating is a two-stage mechanism; for temperatures below the hydrated-to-intermediate phase transition, dehydration is characterized by a homogenous release of protons over the entire oxide lattice, whereas above the transition a preferential desorption of protons originating in the nominally tetrahedral layers is observed. Furthermore, our spectroscopic results point towards the co-existence of two structural phases, which relate to the two lowest-energy proton configurations in the material. The relative contributions of the two proton configurations depend on how the sample is hydrated.

 Please wait while we load your content...
Please wait while we load your content...