Ligand removal from CdS quantum dots for enhanced photocatalytic H2 generation in pH neutral water†
Abstract
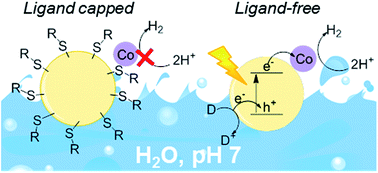
Ligand-free CdS quantum dots were produced by a reactive ligand stripping procedure and employed for photocatalytic H2 evolution in pH neutral solution. The rate of H2 generation of the ‘bare’ quantum dots was 175 times higher than that of the equivalent mercaptopropionic acid-capped quantum dots in the presence of a cobalt co-catalyst and Na2SO3 as a sacrificial electron donor. Under optimised conditions, a turnover number of 58 000 mol H2 per mol Co and 29 000 mol H2 per mol CdS quantum dots was achieved after 88 h of UV-free solar light irradiation (λ > 420 nm, 1 Sun intensity). Ligand removal is therefore a potent method to substantially enhance the photocatalytic performance of quantum dot systems.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...