Revisiting the interpretation of casein micelle SAXS data†
Abstract
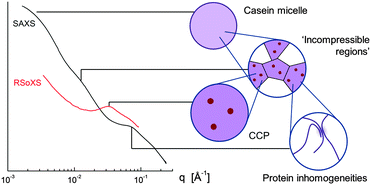
An in-depth, critical review of model-dependent fitting of small-angle X-ray scattering (SAXS) data of bovine skim milk has led us to develop a new mathematical model for interpreting these data. Calcium-edge resonant soft X-ray scattering data provides unequivocal evidence as to the shape and location of the scattering due to colloidal calcium phosphate, which is manifested as a correlation peak centred at q = 0.035 Å−1. In SAXS data this feature is seldom seen, although most literature studies attribute another feature centred at q = 0.08–0.1 Å−1 to CCP. This work shows that the major SAXS features are due to protein arrangements: the casein micelle itself; internal regions approximately 20 nm in size, separated by water channels; and protein structures which are inhomogeneous on a 1–3 nm length scale. The assignment of these features is consistent with their behaviour under various conditions, including hydration time after reconstitution, addition of EDTA (a Ca-chelating agent), addition of urea, and reduction of pH.

 Please wait while we load your content...
Please wait while we load your content...