Shear-induced amyloid fibrillization: the role of inertia†
Abstract
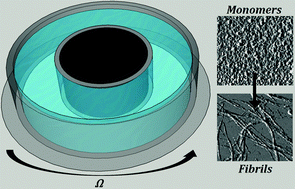
Agitation of protein is known to induce deleterious effects on protein stability and structure, with extreme agitation sometimes resulting in complete aggregation into amyloid fibrils. Many mechanisms have been proposed to explain how protein becomes unstable when subjected to flow, including alignment of protein species, shear-induced unfolding, simple mixing, or fragmentation of existing fibrils to create new seeds. Here a shearing flow was imposed on a solution of monomeric human insulin via a rotating Couette device with a small hydrophobic fluid interface. The results indicate that even very low levels of shear are capable of accelerating amyloid fibril formation. Simulations of the flow suggest that the shear enhances fibrillization kinetics when flow inertia is non-negligible and the resulting meridional circulation allows for advection of bulk protein to the hydrophobic interface.

 Please wait while we load your content...
Please wait while we load your content...