Amyloid-β25–35 peptides aggregate into cross-β sheets in unsaturated anionic lipid membranes at high peptide concentrations
Abstract
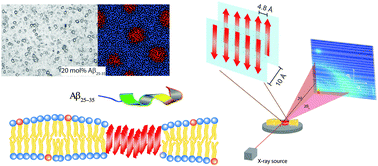
One of the hallmarks of Alzheimer's disease is the formation of protein plaques in the brain, which mainly consist of amyloid-β peptides of different lengths. While the role of these plaques in the pathology of the disease is not clear, the mechanism behind peptide aggregation is a topic of intense research and discussion. Because of their simplicity, synthetic membranes are promising model systems to identify the elementary processes involved. We prepared unsaturated zwitterionic/anionic lipid membranes made of 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine (DMPS) at concentrations of POPC/3 mol% DMPS containing 0 mol%, 3 mol%, 10 mol%, and 20 mol% amyloid-β25–35 peptides. Membrane-embedded peptide clusters were observed at peptide concentrations of 10 and 20 mol% with a typical cluster size of ∼11 μm. Cluster density increased with peptide concentration from 59 (±3) clusters per mm2 to 920 (±64) clusters per mm2, respectively. While monomeric peptides take an α-helical state when embedded in lipid bilayers at low peptide concentrations, the peptides in peptide clusters were found to form cross-β sheets and showed the characteristic pattern in X-ray experiments. The presence of the peptides was accompanied by an elastic distortion of the bilayers, which can induce a long range interaction between the peptides. The experimentally observed cluster patterns agree well with Monte Carlo simulations of long-range interacting peptides. This interaction may be the fundamental process behind cross-β sheet formation in membranes and these sheets may serve as seeds for further growth into amyloid fibrils.
- This article is part of the themed collection: Open access articles from Soft Matter

 Please wait while we load your content...
Please wait while we load your content...