Interaction of partially denatured insulin with a DSPC floating lipid bilayer†
Abstract
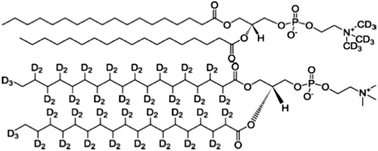
The carefully controlled permeability of cellular membranes to biological molecules is key to life. In degenerative diseases associated with protein misfolding and aggregation, protein molecules or their aggregates are believed to permeate these barriers and threaten membrane integrity. We used neutron reflectivity to study the interaction of insulin, a model amyloidogenic protein, with a DSPC floating lipid bilayer. Structural changes consistent with protein partitioning to the membrane interior and adsorption to a gel phase model lipid bilayer were observed under conditions where the native fold of the protein is significantly destabilised. We propose that the perturbation of the membrane by misfolded proteins involves long term occupation of the membrane by these proteins, rather than transient perforation events.
- This article is part of the themed collection: Open access articles from Soft Matter

 Please wait while we load your content...
Please wait while we load your content...