A new concept for molecular engineering of artificial enzymes: a multiscale simulation†
Abstract
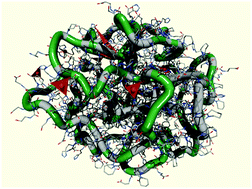
We propose a new concept for the design of artificial enzymes from synthetic protein-like copolymers and non-natural functional monomers which in terms of their affinity for water can be divided into two categories: hydrophobic and hydrophilic. Hydrophilic monomers comprise catalytically active groups similar to those in the corresponding amino acid residues. A key ingredient of our approach is that the target globular conformation of protein-like, core–shell morphology with multiple catalytic groups appears spontaneously in the course of controlled radical polymerization in a selective solvent. As a proof of concept, we construct a fully synthetic analog of serine hydrolase, e.g. α-chymotrypsin, using the conformation-dependent sequence design approach and multiscale simulation that combines the methods of “mesoscale chemistry” and atomistic molecular dynamics (MD). A 100 ns GPU-accelerated MD simulation of the designed polymer-supported catalyst in the aqueous environment provides valuable information on the structural organization of this system that has been synthesized in our Lab.

 Please wait while we load your content...
Please wait while we load your content...