Toward the dynamic phase transition mechanism of a thermoresponsive ionic liquid in the presence of different thermoresponsive polymers†
Abstract
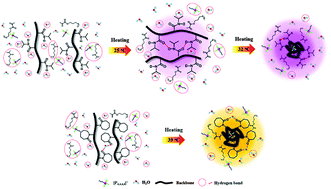
The influence of two thermoresponsive polymers, poly(N-isopropylacrylamide) (PNIPAM) and poly(N-vinylcaprolactam) (PVCL), on the phase transition behavior of a thermoresponsive ionic liquid, tributylhexylphosphonium 3-sulfopropylmethacrylate ([P4,4,4,6][MC3S]), was investigated. An obvious distinction was observed in the LCSTs and morphologies of [P4,4,4,6][MC3S]–PNIPAM and [P4,4,4,6][MC3S]–PVCL aqueous solutions, indicating their large differences in dynamic transition processes. In general, PNIPAM can “break” the water structure of [P4,4,4,6][MC3S] to decrease the transition temperature, while PVCL can “make” the water structure to increase it. Surprisingly, [P4,4,4,6][MC3S] has an unusual over-hydration behavior before dehydration while PNIPAM experiences a two-step transition process in [P4,4,4,6][MC3S]–PNIPAM aqueous solution, which has never been reported so far. Further studies revealed that the formation of strong intra-/inter-molecular hydrogen bonds C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯D–N in PNIPAM is the driving force for the LCST phenomenon of [P4,4,4,6][MC3S]–PNIPAM solution, while it is the [P4,4,4,6][MC3S] that dominates the phase separation of [P4,4,4,6][MC3S]–PVCL solution.
O⋯D–N in PNIPAM is the driving force for the LCST phenomenon of [P4,4,4,6][MC3S]–PNIPAM solution, while it is the [P4,4,4,6][MC3S] that dominates the phase separation of [P4,4,4,6][MC3S]–PVCL solution.

 Please wait while we load your content...
Please wait while we load your content...