Bond fission in monocationic frameworks: diverse fragmentation pathways for phosphinophosphonium cations†
Abstract
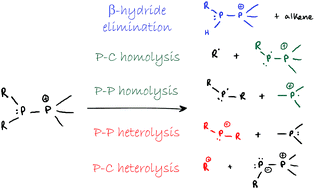
A series of phosphinophosphonium cations ([R2PPMe3]+; R = Me, Et, iPr, tBu, Cy, Ph and NiPr2) have been prepared and examined by collision-induced dissociation (CID) to determine the fragmentation pathways accessible to these prototypical catena-phosphorus cations in the gas-phase. Experimental evidence for fission of P–P and P–E (E = P, C) bonds, and β-hydride elimination has been obtained. Comparison of appearance potentials for the P–P bond dissociation fragments [R2P]+ (P–P heterolysis) and [PMe3]+˙ (P–P homolysis) shows that heterolytic P–P cleavage is more sensitive than P–P homolysis towards changes in substitution at the trivalent phosphorus center. The facility of β-hydride elimination increases with the steric bulk of R in [R2PPMe3]+. A density functional theory (DFT) study modelling these observed processes in gas-phase, counterion- and solvent-free conditions, to mimic the mass spectrometric environment, was performed for derivatives of [R2PPMe3]+ (R = Me, Et, iPr, tBu, Ph and NiPr2), showing good agreement with experimental trends. The unusual observation of both homolytic and heterolytic cleavage pathways for the P–P and P–C bonds reveals new insight into the fundamental aspects of bonding in monocations and undermines the use of simplistic bonding models.

 Please wait while we load your content...
Please wait while we load your content...