Linker-free incorporation of carbohydrates into in vitro displayed macrocyclic peptides†
Abstract
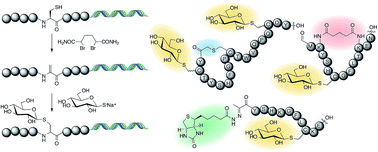
We report a strategy for efficient post-translational modification of a library of ribosomally-translated peptides by activation and elimination of cysteine to dehydroalanine then conjugate addition of a range of exogenous thiols, with an emphasis on carbohydrates. These reactions are selective for cysteine, and do not interfere with amplification of the nucleic acid component of an mRNA-displayed peptide. Furthermore, these reactions are shown to be compatible with two different macrocyclisation chemistries, and when applied to a peptide containing an N-terminal cysteine give a ketone that can be functionalised in an orthogonal manner. This new strategy can overcome a limitation of ribosomal translation, providing a means to incorporate untranslatable groups such as carbohydrates in amino acid side chains, and will allow for the ribosomal generation of glycopeptides, requiring only the introduction of a free thiol in the molecule to be incorporated. In combination with in vitro selection techniques, this strategy is envisaged to allow the discovery of biologically-active glycopeptides with a near-natural, but hydrolytically stable, thioglycosidic bond.


 Please wait while we load your content...
Please wait while we load your content...