Superior anion induced shuttling behaviour exhibited by a halogen bonding two station rotaxane†
Abstract
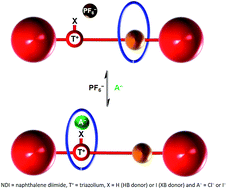
Two bistable halogen and hydrogen bonding-naphthalene diimide [2]rotaxanes have been prepared and the system incorporating a halogen bond donor anion recognition site is demonstrated to exhibit superior anion induced translational motion of the macrocyclic wheel component relative to the hydrogen bonding analogue. Proton NMR spectroscopy is used to estimate the percentage occupancies of the macrocycle at the respective station and importantly indicates that the halogen bonding rotaxane displays superior positional integrity in competitive protic solvent media as a consequence of strong halogen bond–halide anion binding interactions.
- This article is part of the themed collection: In celebration of Paul Beer’s 60th Birthday


 Please wait while we load your content...
Please wait while we load your content...