Photoelectrochemical hydrogen production in water using a layer-by-layer assembly of a Ru dye and Ni catalyst on NiO†
Abstract
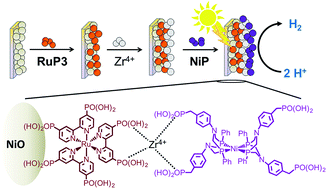
Capture and conversion of sunlight into the storable energy carrier H2 can be achieved through photoelectrochemical water splitting using light-absorbing cathodes and anodes bearing H2 and O2 evolving catalysts. Here, we report on the development of a dye-sensitised p-type nickel oxide (NiO) photocathode with a hexaphosphonated Ru(2,2′-bipyridine)3 based dye (RuP3) and a tetraphosphonated molecular [Ni(P2N2)2]2+ type proton reduction catalyst (NiP) for the photoreduction of aqueous protons to H2. A layer-by-layer deposition approach was employed, using Zr4+ ions to link the phosphonate units in RuP3 and NiP in a supramolecular assembly on the NiO photocathode. This approach keeps the dye in close proximity to the catalyst and semiconductor surface, but spatially separates NiP from NiO for advantageous electron transfer dynamics. The NiO|RuP3–Zr4+–NiP electrodes generate higher photocurrents and are more stable than photocathodes with RuP3 and NiP co-immobilised on the NiO surface in the absence of Zr4+ cations linking dye and catalyst. The generation of H2 with the NiO|RuP3–Zr4+–NiP hybrid electrode in pH 3 aqueous electrolyte solution during irradiation with a UV-filtered solar light simulator (λ > 400 nm, 100 mW cm−2, AM1.5G) has been confirmed by gas chromatography at an underpotential of 300 mV (Eappl = +0.3 V vs. RHE), demonstrating the potential of these electrodes to store solar energy in the chemical bond of H2.
- This article is part of the themed collections: In celebration of Kazunari Domen’s 65th birthday, 2018, ISACS17: Challenges in Chemical Renewable Energy, Global Energy Challenges: Solar Energy and Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...